Serpentinization Makes Jade
Today Dr. Josh Stout discusses how Serpentinization makes jade - and how it will save the world and may be the origin of life.

How it will save the word and may be the origin of life
Please scroll down for links below the transcript. This is lightly edited AI generated transcript and there may be errors.
Josh - Nov 2 0:07
Hello. It's November 2nd. This is Dr. Stout, and I would like to talk about how serpentinization makes the jade for me, but also may save the world and maybe the origin of life itself. This is sort of a technical supplement. I'm going to be talking about some things outside of my specialty, my geology, chemistry, a range of really specialized technical information. That is not my usual topic, but I'm really interested in these subjects, obviously, because I like to carve jade and also because I'm interested in the origin of life and saving the world. So what is serpentinization? Serpentinization is when water comes down from the surface and encounters a batch of iron containing rocks in the sort of a set set of conditions of decent amount of pressure and a high temperature, but not too high. And then the rock, the iron in the rock becomes oxidized and the oxygen is coming from the water. And so it releases hydrogen. And so this was in the news recently where a new drill hole in France that was looking for natural gas actually found hydrogen instead. And so this is very exciting. And it may well be the saving of the world because it is clean hydrogen that doesn't need other forms of energy to produce it. Obviously, there's a little bit of energy in getting it out of the ground in the first place. But other than that, it is the perfect green energy source. When you burn hydrogen, it just combined with oxygen to make water again. So there's no pollution, there's no particulate matter. It is just about the perfect fuel. It has a couple of problems with storage and things like that. It's very, very bulky. But in terms of just a perfect fuel, it's really hard to beat the cleanness of hydrogen. So why am I so interested in serpentinization?
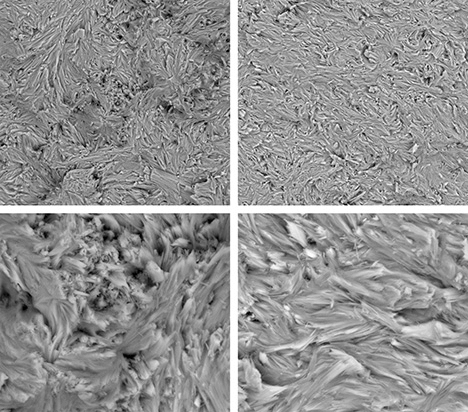
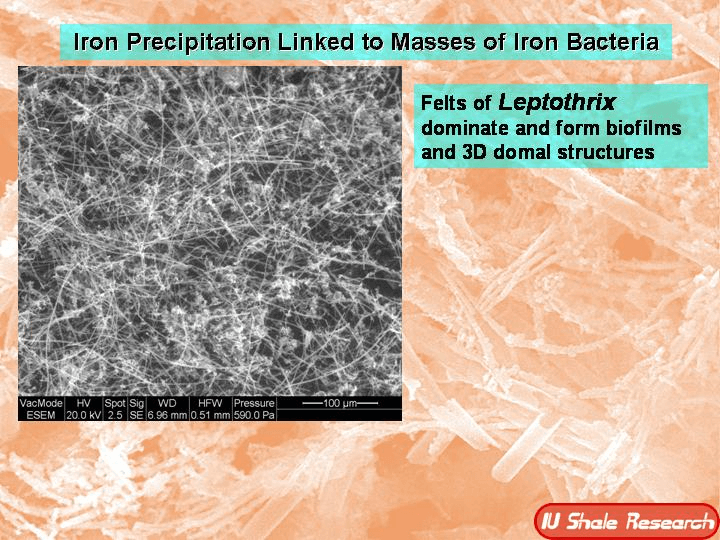
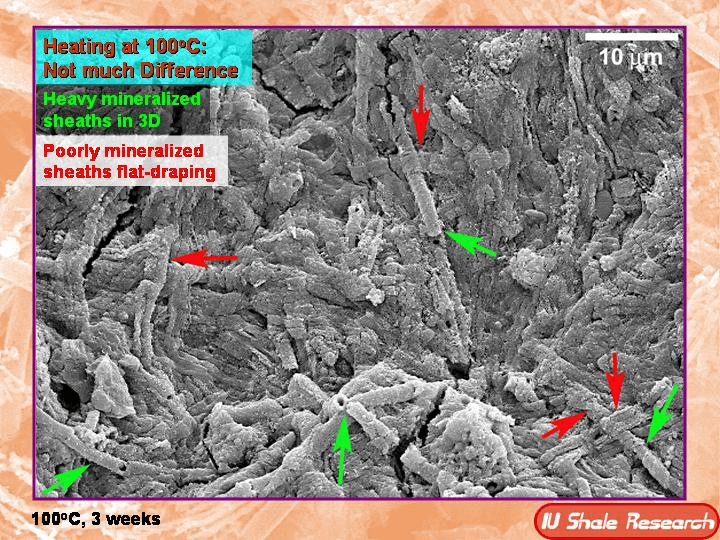
It happens really anywhere. Water can encounter iron, particularly in a silicates rock form, and it's mimicking the same kind of actions that happen when life in a marsh or in a deep sea area also has iron encountering water. So microbes in low oxygen or no oxygen environments can actually use iron and combine it in various ways with water as a form of energy. Sometimes this is an abiotic process that releases hydrogen that the bacteria can then use themselves. So it's also a form of food for the bacteria if it's abiotic. But I suspect in many cases it is a biotic mediated process that is changing iron f e to oxidized forms first, f, e two, and then fe3. So the two forms of ferric and then ferrous iron that have very different looks and the fair ferric iron, the fe2 is red, while ferrous iron becomes fe3 and is, is, is green. And that is what the green in my jade is. So this is a process that is happening really everywhere that there is both water and iron and the bacteria are using these as energy forms. And the interesting thing is there is a competition between abiotic processes and life and any energy that's just there to be scavenged. Life would like to use it, but sometimes it just, you know, things burn on their own without life being involved in oxidizing something or moving electrons around. So in the in the deep sea, it turns out and in in marshes and in where Yes, again everywhere this is happening, it turns out that under slightly acidic conditions, bacteria can often produce the abiotic sources. And then what they'll do is they'll turn F2 to F3. The part I'm particularly interested in and in the production of F3, the iron becomes less toxic to the bacteria, it becomes less soluble and it ends up ends up being excreted as a iron oxide and actually does these really weird things. It makes these long braided strands as the bacteria get rid of the excess iron. So as they're essentially eating iron there, there, there, they're iron poop, looks like long threads and this forms bacterial mats. You might have actually seen this sometimes streams in the summertime as they're running low. I will be a little bit acidic and you'll start to see a sort of red slime forming on the base bottom of the stream that is actually iron oxidizing bacteria making that reddish slime. But in other conditions that would be green. Now, obviously, you know, in a in a stream on the surface, you're going can have enough oxygen that it can form other other forms of of of iron oxide. And then the green one. But the green one happens preferentially under very deep conditions, preferentially at about what is about 18 K bar, which I had to look up at, that's about 18,000 times 14 and a half pounds is the actual pressure comparing it to sort of PSI. So pounds per square inch it would be 18,000 times 14 and a half to give you a total pressure and happens about 300 degrees centigrade. So it's an eight K bar and 300 C, so it's hot, but not incredibly hot. And these are temperatures that life might actually be able to exist at those pressures. Water doesn't boil. So the the cells wouldn't burst. And if life is able to outcompete the abiotic factors under these circumstances, it's a very useful form of energy. So there is a ton of energy released in the process of oxidizing iron. It's a very exothermic reaction. And one of the interesting aspects of it is that as the reaction occurs, it releases the heat to the to more or less the temperature that it needs. And obviously there's different depths where you can find the right temperature, but it makes its own heat and it releases about 300 degrees worth of heat and it delivered about 300, 350 degrees. So if it gets too hot, the process slows down. And if it cools down, the process slows down. So there's an optimal temperature, but it's able to simply because the process exists, the process will expand the region of that optimal temperature by producing its own heat. So it's a really interesting reaction that has similarities to the way bodies work, right? So that bodies are working at a particular temperature at which they work best to produce that particular temperature. It's it's a homeostasis. And there is also a changing from one thing to another and changing the iron into the iron three and changing taking the oxygen out of the water and releasing hydrogen. That's metabolism. So there's many aspects of this that really resemble life and to me are almost a, you know, interesting way of thinking about how the earth is in a certain way alive. And it might actually be life, it might actually be bacteria helping this process. And one of the sort of interesting side things about this is serpentinization making serpentine involves a sort of fibrous matted look to the the resulting minerals, particularly jade. If you look at jade, it looks like almost like felt like like fibers that have been laid down flat and all tangled together. And that's what gives Jade its toughness. And if you look at bacterial mats that have been oxidizing iron as these threads come out of the bacteria, they also form these kinds of matted kinds of felted looks. And I find the the similarity somewhat compelling. I mean, obviously I can't prove anything because they happen to both have these thin matted threads. But it's the kind of thing that is at least, you know, circumstantial evidence that these are bacterial mediated reactions that might be producing the very rocks I carve, which I find very interesting.
The the matting process produces iron that is pretty resistant. It's insoluble. And so these would make excellent fossils. And one of one of the ideas about Jade is that it's often what's called metal somatic. It's a wonderful world word meta, meaning, you know, changing or going beyond and somatic meaning body and it's when one rock replaces another rock. And so Jade is often seen as being metal, somatic and, and replacing other other other other things. And it may well have been associated with this kind of iron matting or a little further out, a little more science fictiony, the silica in jade and the iron in jade together might be some sort of I bacterial or living, let's say, form of production archaea are also down there doing some of these things and there may well be other forms of life similar to life that we know we're not, not, not, not to science fiction, but, you know, DNA based life with cells that we don't even know about. Right. So archaea were only found in the seventies. Now we're realizing they're everywhere. But there could well be things that are down there that we don't know about it. Those pressures and those temperatures are even more science fiction. There might be some sort of thing that's involving transformation of silica itself. You know, back in the early days of science fiction, that was often one of the speculations that there was some sort of silica life. I'm not saying there is Everything I see could easily be done by bacteria, but it's kind of fun to think about that.
The very beginnings of of of life may have involved some some aspects of silica chemistry. I haven't really talked about that part of it or why why I keep mentioning this at the beginning of life, not just something producing free hydrogen that'll give us clean energy, but the idea is that when this hydrogen is produced, it's often produced both in coastal margins and at sea for seafloor spreading areas and near hydrothermal vents. And when this hydrogen is produced, it's a rich source of food for bacteria. And so chemo, synthetic bacteria, it's bacteria that are able to eat chemicals as opposed to, say, photosynthetic bacteria that are eating life sorry, heating light the chemo synthetic bacteria are using I generally assumed to be abiotic produced chemicals such as hydrogen as as direct sources of energy. I'm wondering if this hydrogen isn't also being produced by other other living organisms. And that would that would go along with a lot of the way life works, right? Life tends to make its own habitat, right? It life makes soil that plants can grow in, plants feed the animals, etc.. So it life makes the habitat for itself. Perhaps the hydrogen that is being produced is actually being produced by bacteria that I think there are some cases where that is definitely the case and that these this hydrogen then provides a ecosystem for a whole wealth of other bacteria and then higher forms of life above that. And so one speculation is that these oases of of of life may have provided a way for living chemistry to develop in the very early days of of of Earth at the very beginning, the Earth was not habitable by anything, by any any forms of life. And there were, you know, asteroids hitting all the time, comets hitting all the time. It would have been a, you know, a terrible place for for life to evolve. But having a protective blanket of an ocean on top of you would have would have allowed life to exist in a somewhat more stable environment. The problem is it's not exposed to any sunlight, so there wouldn't be any forms of energy to be used that we normally think of. As you know, everything is based on on the sun's energy. So it would have to be the chemo, synthetic energy. And so one of the leading theories for the beginning of life is that it happened at these underwater sites. Sometimes people have, say, hydrothermal vents, or it could be ocean seafloor spreading areas where it was it was stable and gave a place for life to develop over time. Another really interesting thing is there's been periods, periodic times when there was the life, the earth was covered with ice and snow, and there would have been in any place for life to exist on the surface. And during these times, the hydrothermal vent communities and these chemo's synthetic vent communities may well have been a way for life to survive during these times when Earth would have not been habitable in any way. That was ice from pole to pole. So it's really interesting to think about how these things are connected. Serpentinization, the the the formation of serpentine releases. Naturally hydrogen, it's about somewhere around a half, a half a gram per kilo of of serpentine, of hydrogen that gets produced and that's a tonne. Right. So rock is heavy and for every kilo of it you're going to get a half, a half gram of hydrogen, which is a tremendous, tremendous production rate. So this is, this is, this is a very hopeful thing to think about. And it's making jade for me, which is just wonderful. The chemistry is is is very involved. I definitely do not understand all the aspects of it, but it's really neat to see all the bits that I that I can can understand when I, when I notice something. You know, for example, one of the side chains is making something called magnesite, not magnetite. And I would have thought that magnesite was almost the same thing as magnetite. Magnetite is another oxidized form of of iron, perhaps produced by bacteria. There are bacteria that definitely produce magnetite, but magnesite is a magnesium carbonate. And so magnesite when it forms is going to be taking CO2 out of out of the water. So this may be a way that global warming can be addressed as well, that the carbonate rocks may be a way to sequester CO2. So the entire process is is is wonderfully interesting. And, you know, in the news today and as related to evolution in general, the development of life, the beginning of life. And I thought it would be worth talking about a little bit as sort of a side technical note before we get back to some of my other other discussions. Thank you.
HIDDEN HYDROGEN
Does Earth hold vast stores of a renewable, carbon-free fuel?
A version of this story appeared in Science, Vol 379, Issue 6633.
Characterization of the spontaneously recharging natural hydrogen reservoirs of Bourakebougou in Mali
From Nature.com July 22, 2023

Theme Music
Theme music by
sirobosi frawstakwa







